Nearly 1.35 billion people in India are desperately waiting for a vaccine. While the government has been putting in a plan to efficiently vaccinate the large population, the approval of the AstraZeneca coronavirus vaccine has been a piece of promising news. This is particularly important for India as it paves the way for the vaccination of its population. Various vaccines like one by Pfizer and Moderna have already been approved but the logistics involved make it very difficult for India to distribute its huge population. These mRNA based vaccines required deep cold storage of nearly -70° C, making it incredibly difficult to reach masses in India.
AstraZeneca said the authorization was for a two-dose regime, and that the vaccine had been approved for use for emergency supply. Britain has already ordered 100 million doses of the vaccine.
The vaccine is yet to be approved in India, however, its approval in the U.K makes it far easier to get authorization for emergency use in India.
“The government has today accepted the recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) to authorize Oxford University/AstraZeneca’s COVID-19 vaccine for use,” the health ministry in U.K said.
About the AstraZeneca Vaccine
The Oxford-AstraZeneca vaccine was designed in the first months of 2020, tested on the first volunteer in April, and has since been through large-scale clinical trials involving thousands of people.
The incredible speed at which it has been developed and tested was almost unconceivable before the current COVID-19 Pandemic. The decision to approve the vaccine was taken under Regulation 174 of the Human Medicine Regulations 2012, which enables rapid emergency regulatory approvals to address significant public health issues such as a pandemic. This is the first authorization for this vaccine.
It is, however, the second vaccine to be approved for emergency use in the UK after the Pfizer-BioNTech vaccine was given the go-ahead in December.
AZD1222 (generic name of the vaccine) was co-invented by the University of Oxford and its spin-out company, Vaccitech. It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body.
[rb_related title=”You May Also Like” total=”2″]
The interim analysis for efficacy was based on 11,636 participants accruing 131 symptomatic infections from the UK and Brazil Phase III trials conducted by Oxford University. As announced on 23 November 2020, the primary efficacy endpoint based on a pooled analysis showed that the vaccine was 70.4% (confidence interval: 54.8% to 80.6%) effective at preventing symptomatic COVID-19 occurring more than 14 days after receiving two doses of the vaccine. A secondary efficacy endpoint of prevention of severe disease demonstrated no cases of severe infections or hospitalizations in the vaccine group.
Importance for India
Oxford-AstraZeneca vaccine will lead to a significant improvement in vaccination as it is cheap and easy to mass-produce. More importantly, it can be stored in a standard fridge – unlike the Pfizer-BioNTech jab which needs ultra-cold storage at -70C – so it will be far easier to get the Oxford vaccine to care homes and GP surgeries. Priority groups for immunization – including the elderly, care home residents, and health and care workers – have already been identified.
The approval is a big boost to Serum Institute, which is among the top three vaccine makers in the race to help India vaccinate its massive population. It has already applied for regulatory approval with the Drug Controller General of India but is yet to get the mandatory approval for its vaccine. The approval from the U.K will make the approval easier in India.
The world’s largest vaccine maker by volume, Serum Institute has already manufactured 50 million doses of Covishield vaccine on at-risk funding. Overall, the vaccine giants aim to develop 3.2 billion doses of coronavirus vaccines at its facilities in India.
SII chief executive Adar Poonawalla had earlier said the vaccine candidate could get approval by next week in India and might be rolled out for the masses in January. “The company is ramping up capacity every week,” he said.
For the U.K, the new vaccine approval comes after Public Health England said the country was facing “unprecedented” levels of infections, n the backdrop of a new coronavirus strain, and health officials in parts of Wales, Scotland, and the south of England voiced concerns about the increasing pressure on the NHS.
How does the vaccine work?
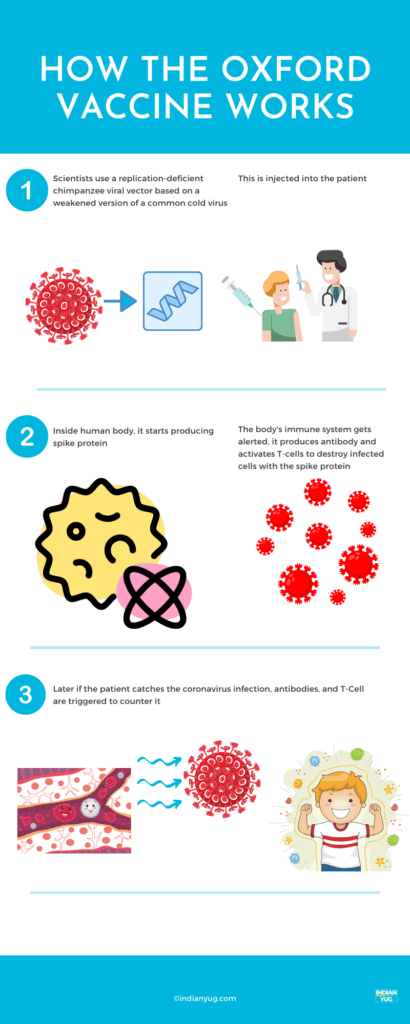
The vaccine is a genetically modified common cold virus that used to infect chimpanzees.
It has been altered to stop it from causing an infection in people and to carry the blueprints for part of the coronavirus, known as the spike protein.
Once these blueprints are inside the body they start producing the coronavirus’s spike protein, which the immune system recognizes as a threat and tries to fight it off. Later, when the immune system comes into contact with the virus for real, it already has a memory of knows what to do.