A review by the EU’s medicines regulator on Thursday has concluded that the Oxford-AstraZeneca Covid-19 vaccine is “safe and effective”, and its benefits outweigh the risks.
The European Medicines Agency (EMA) found the vaccine was “not associated” with a higher risk of blood clots. However, it said that it will continue to study the possibility of such links.
The World Health Organization (WHO) on Thursday called on countries to continue using the vaccine.
The concerns about the AstraZeneca vaccine arose after 13 EU states decided to suspend its use and there was widespread concern over the speed of vaccination drive which was already impacted by supply shortages across EU.
Much of Europe is struggling to contain a surge in coronavirus cases. The WHO is also investigating the cases of blood clotting-related side effects of the vaccine and is due to release the results of its review into the vaccine’s safety on Friday.
The EMA investigation focused on a small number of cases of unusual blood disorders. In particular, it was looking at cases of cerebral venous thrombosis – blood clots in the head.
Why was the use of vaccines suspended in some EU countries?
Earlier, thirteen EU countries suspended use of the vaccine, after reports of a small number of cases of blood clots among vaccine recipients in the region.
On Monday, the three largest EU members – Germany, France, and Italy – said they were awaiting the results of the EMA investigation before deciding whether to resume their rollout of the jab.
They said they had opted to pause their use of the drug as a “precautionary measure”.
Denmark, the first to suspend the shot last week after reporting “highly unusual” symptoms in a 60-year-old woman who died from a blood clot
“There were a few very unusual and troubling cases which justify this pause and the analysis,” French immunologist Alain Fischer, who heads a government advisory board, told France Inter radio. “It’s not lost time.”
In Germany, the health ministry also pointed to a small number of rare blood clots in vaccinated people when justifying its decision. It postponed a summit on extending the vaccine rollout ahead of the EMA’s announcement.
Other countries, such as Austria, halted the use of certain batches of the drug, while Belgium, Poland, and the Czech Republic were among those to say they would continue to administer the AstraZeneca vaccine.
It is up to individual countries to decide whether to follow the EMA recommendations.
Decisions to stop rollouts of the AstraZeneca vaccine were criticized by some politicians and scientists.
A spokeswoman for Germany’s opposition Free Democrats said the decision had set back the country’s entire vaccination rollout. German Greens health expert Janosch Dahmen, meanwhile, argued that authorities could have continued using the drug.
Dr Anthony Cox, who researches drug safety at the UK’s University of Birmingham, told the BBC it was a “cascade of bad decision-making that’s spread across Europe”.
Many Europeans, however, appear to feel the move was justified — a poll on Wednesday showed 54% of Germans believed the government had taken the right decision. But with Europe’s vaccination campaign already lagging far behind that of countries such as Israel, the US and the UK, politicians are now in a hurry to resume AstraZeneca shots.
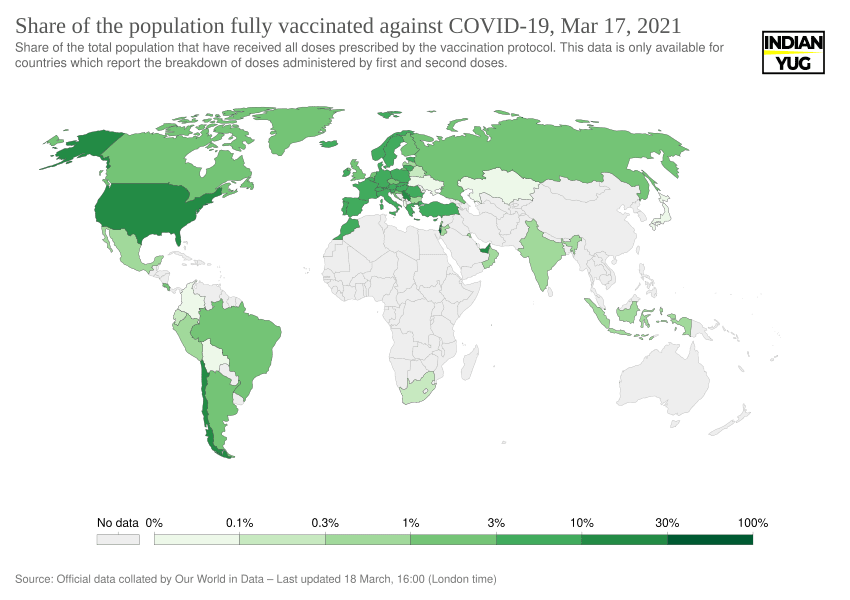
The EU bloc has administered only 11 shots so far for every 100 residents, while Israel – a world leader in vaccination – has given 108 doses, according to Our World in Data.
[rb_related title=”You May Also Like” total=”2″]
What has AstraZeneca said about its vaccine?
The company has been asserting that there is no evidence of an increased risk of clotting due to the vaccine.
It said it had received 37 reports of blood clots out of more than 17 million people vaccinated in the EU and UK as of 8 March.
These figures were “much lower than would be expected to occur naturally in a general population of this size and is similar across other licensed Covid-19 vaccines”, it said.
Professor Andrew Pollard, director of the Oxford vaccine group which developed the Oxford-AstraZeneca jab, told the BBC on Monday that there was “very reassuring evidence that there is no increase in a blood clot phenomenon here in the UK, where most of the doses in Europe have been given so far”.
British Health Secretary Matt Hancock this week urged people to “listen to the regulators” and to “get the jab”.
What does the EMA report say?
EMA’s safety committee, PRAC, concluded its preliminary review of a signal of blood clots in people vaccinated with COVID-19 Vaccine AstraZeneca at its extraordinary meeting of 18 March 2021. The Committee confirmed that:
- The benefits of the vaccine in combating the still widespread threat of COVID-19 (which itself results in clotting problems and maybe fatal) continue to outweigh the risk of side effects;
- The vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it;
- There is no evidence of a problem related to specific batches of the vaccine or to particular manufacturing sites;
- However, the vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it to clot) with or without bleeding, including rare cases of clots in the vessels draining blood from the brain (CVST).
These are rare cases – around 20 million people in the UK and EEA had received the vaccine as of March 16 and EMA had reviewed only 7 cases of blood clots in multiple blood vessels (disseminated intravascular coagulation, DIC) and 18 cases of CVST. A causal link with the vaccine is not proven, but is possible and deserves further analysis.
Share of the population fully vaccinated against COVID-19
Experts say that as with all medicines, regulators must seek to balance the benefits of any vaccine with any potential risks. In this case, they argue, the calculation must take into account the risk from Covid-19 – which is clearly many multiples higher.